Professor Zhiqiang Li and his research team at the Biomedical Sciences Institute, Medical College, have published a research article in Biological Psychiatry (https://doi.org/10.1016/j.biopsych.2024.06.015), a leading journal in neuroscience and psychiatry. The paper, titled "The Contribution of Mosaic Chromosomal Alterations to Schizophrenia," investigates the crucial role of early developmental somatic copy number variations (sCNVs) in schizophrenia.
This comprehensive study utilized large-scale whole-genome genotyping data from blood samples of the Chinese population, complemented by blood and post-mortem brain tissue genotyping data from European cohorts. The rigorous analysis led to the creation of a mosaic chromosomal alterations (mCAs) map, revealing the critical involvement of sCNVs in schizophrenia. The study's co-first authors include master's student Kaihui Chang, Dr. Xueming Jian, Dr. Chuanhong Wu, Dr. Chengwen Gao, and undergraduate student Yafang Li. Qingdao University served as the primary institution, with Professors Zhiqiang Li and Yongyong Shi as the corresponding authors.
Schizophrenia is a complex, severe mental disorder characterized by abnormal mental activities, cognitive dysfunction, and memory impairment. With a prevalence of approximately 1% and high disability rates, schizophrenia places a significant burden on patients, their families, and society. Genetic factors are crucial in the development of schizophrenia, contributing up to 80% heritability. However, the challenge of "missing heritability" has impeded progress in genetic research on schizophrenia.
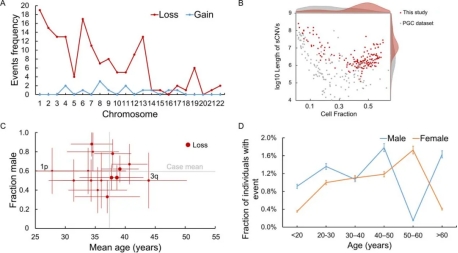
Burden of sCNVs in Schizophrenia Among the Chinese Cohort
The research team analyzed DNA genotyping data from 38,537 Chinese participants using the innovative high-sensitivity MoChA algorithm to identify sCNVs associated with schizophrenia. This data included 9,715 schizophrenia patients and 28,822 controls. Additionally, the team integrated data from the Psychiatric Genomics Consortium (PGC), consisting of European cohorts (12,834 cases and 11,648 controls), and post-mortem brain tissue genotyping data from 936 subjects (449 cases and 487 controls).
The findings revealed a significantly higher rate of somatic genome copy number loss (deletions) in schizophrenia patients compared to controls (1.00% vs. 0.52%; odds ratio (OR) = 1.91; 95% confidence interval (CI), 1.47–2.49). Moreover, as the cellular fraction threshold increased, the OR also increased (from 1.91 to 2.78), underscoring the association of several sCNVs with schizophrenia. These sCNVs are implicated in key pathways related to schizophrenia pathogenesis, including synaptic function, neurotransmitter release, and glutamatergic and GABAergic signaling, which are essential for maintaining the balance between excitatory and inhibitory neurotransmission in the brain.
The study underscores the significant role of mosaic chromosomal alterations (mCAs) in the pathogenesis of schizophrenia, marking a substantial advance in understanding the genetic basis of this disorder and paving the way for future studies and potential therapeutic developments.
The Biomedical Sciences Institute places a strong emphasis on fostering innovation and research capabilities in undergraduate students. This commitment is evident in the involvement of undergraduate clinical medicine students in top-tier research publications. Since the launch of the clinical medicine undergraduate training program in 2020, this study marks the second article published in Q1 journals in the CAS journal ranking list with active participation from undergraduate students within three years.